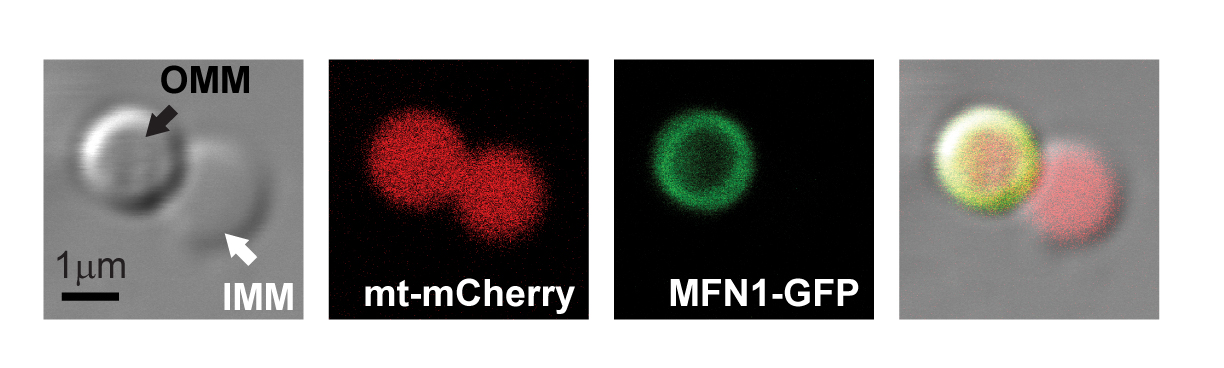
RESEARCH
As heart failure progresses, rewiring of metabolism leads to a global decline in ATP synthesis. In studying heart failure, we focus on calcium signaling, since it is critical for cardiac contraction and metabolism. We hope to define the molecular pathways that control mitochondrial calcium signaling and target these for novel therapeutics.